CDMO PROFILE
Strong Scientific Lead

Lead Scientist
Post Doc - Thomas Jefferson University, USA. PhD in Mol. Bio. - IICB, Jadavpur Univ. Focuses on mammalian and microbial technology developments, Cloning and Protein Engineering.

Senior Scientist
PhD (Tech.), Anna University, M. Tech Biotech, Anna University, India. Expert in bioprocess engineering and protein purification. With Epygen since 2012 and played key role in technology transfers

Head - Regulatory
PhD Biopharmaceuticals, VIT, Vellore. M.Pharm. Ramachandra Med. Coll., India. Has international Biopharma experience and handles Epygen’s Global regulatory framework.

Chief Scientific Advisor
Scientist who invented India’s first bio-therapeutic drug and fourth gen molecule for Ischemic Stroke. PhD from IISC Bangalore and Post Doc from Univ. of California and Albert Einstein Coll. of Med., NY.
Capabilities and Capacities for CDMO services
Epygen offers an end-to-end Biological service from clone till vialing for intra/extra cellular therapeutic proteins. We are capable to scale up and manufacture sensitive proteins undergoing cell lysis, solubilisation, refolding and range of purification processes entailing chromatography and membrane separations
R&D and Analytical Development
- Equipped with latest Molecular Biology, Protein Engineering and Bioprocess tools. In-house analytical, bioassay & stability analysis, meeting latest guidelines
- Equipped with latest Molecular Biology, Protein Engineering and Bioprocess tools. In-house analytical, bioassay & stability analysis, meeting latest guidelines
Scale-Up and Manufacturing DS
- Scale-up of Fermentation and Purification processes upto 150 L catering to pre-clinical, clinical and commercial requirements
- Capable of handling PEGylation
Fill & Finish of DP
- Aseptic filling & Packaging of Biologics in vials and in-house lyophilization capabilities
- On-site Characterization, QC, Testing and release, Specialized Bologics storage.
Overall Biologics development snapshot:
- Epygen can support and develop products and processes from Clone, Pre-clinical and Clinical, till commercial stages, abiding highest global standards
- 1. Clone selection, Cloning and Clone QC, Cell Banking
- 2. Analytical development & qualification
- 3. Scaling up of Fermentation, optimization & development
- 4. Scaling up of DSP - Lysis, Centrifugation, Ultra- Dia Filtration, TFF
- 5. Large scale IEX, HIC, Affinity Chromatography, Endotoxin removal, Polishing
- 6. Fill & Finish Formulation development of injectables & Stability studies (ICH compliant).
- 7. Analytical method development and validation
- 8. cGMP Regulatory batches USP & DSP 20,50,100 Lt scales
- 9. Pre-Clinical Studies / Complete Biological and Physicochemical Characterization
- 10. Tech. transfer for commercial Mfg
- 11. Process validation at Mfg. scale
Technologies available for transfer
Filgrastim (recombinant G-CSF) Technology
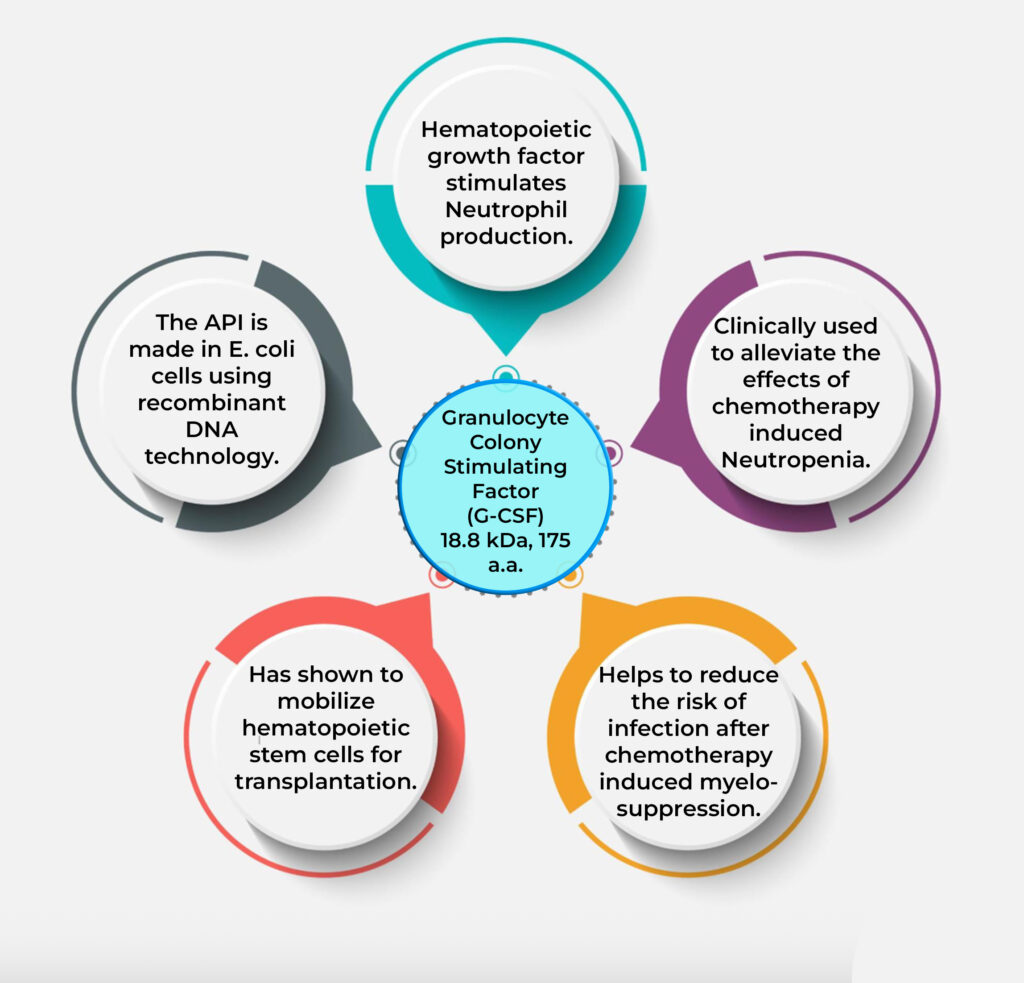
Epygen Biotech employs recombinant DNA technology in E. coli to produce Filgrastim Biosimilar (recombinant G-CSF), optimizing a 175-amino-acid, non-glycosylated protein (18.8 kDa). An advanced high-density fermentation and precision refolding techniques, coupled with rigorous chromatographic purification ensures high-purity output. This scalable, cost-effective process targets chemotherapy-induced neutropenia and stem cell mobilization, meeting stringent quality standards. Technology is available for Bench, Pilot and CGMP scale transfer.
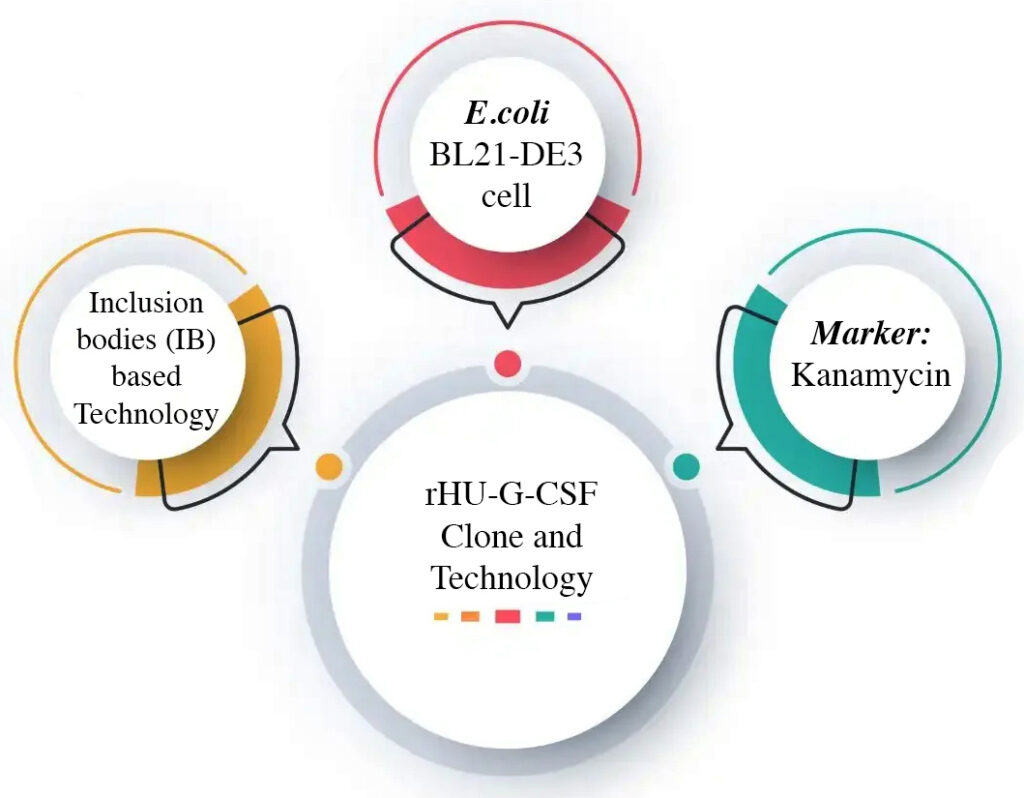
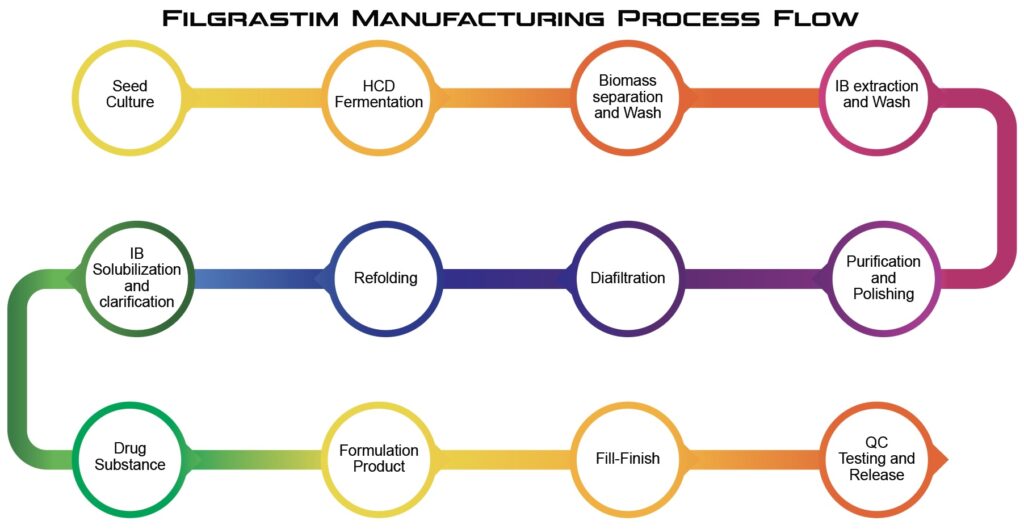
At Epygen Biotech, we offer technology for Filgrastim, a recombinant G-CSF, using *E. coli*-based systems at our facility near Mumbai, India. The process entails cloning the G-CSF gene into *E. coli* for enhanced protein expression. High-cell-density fermentation in bioreactors to optimize yield, followed by cell lysis to harvest Filgrastim-containing inclusion bodies (IB). These are denatured, refolded, and purified using advanced chromatography (e.g., ion-exchange, hydrophobic interaction) to achieve high purity. The protein is formulated with stabilizers and excipients and finally filled into vials or syringes under sterile cGMP conditions. Epygen’s innovative, cost-effective approach ensures affordable, high-quality Filgrastim producing technology for Oncology, meeting global regulatory standards.
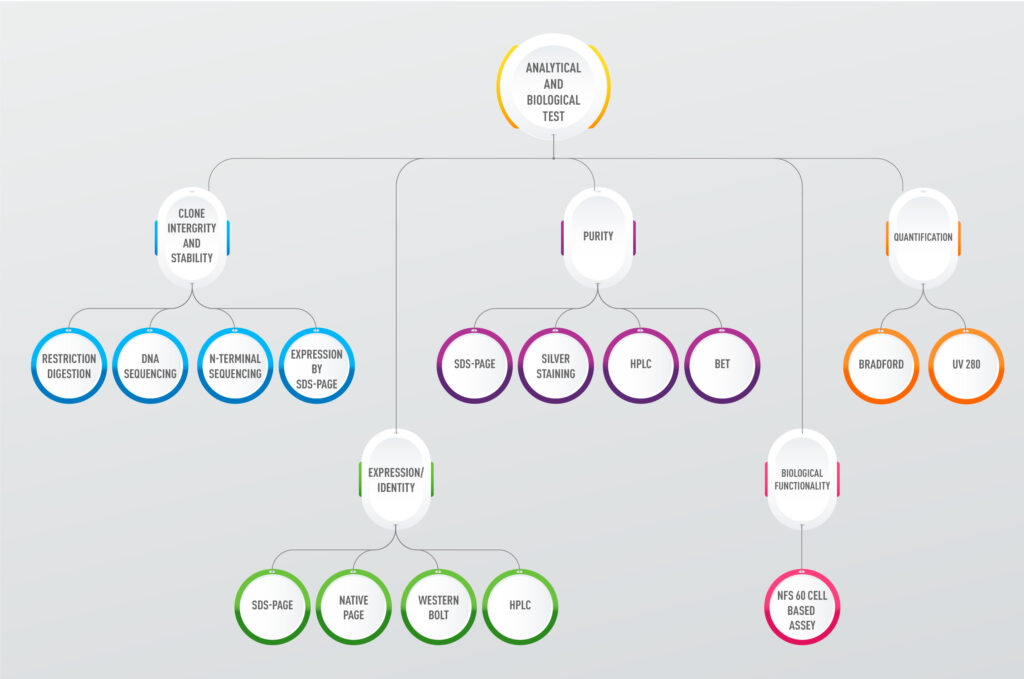
Epygen Biotech employs advanced analytical and biological testing to ensure the quality and safety of its biopharmaceuticals, including recombinant proteins and biosimilars for oncology and cardiovascular diseases. Analytical tests, such as HPLC and mass spectrometry, verify purity, stability, and potency. Biological testing, including cell-based assays, confirms bioactivity and safety. Conducted in multiple facilities, these tests ensure compliance with global regulatory standards, supporting Epygen’s mission to deliver affordable, high-quality therapeutics.
Harvest and IB processing
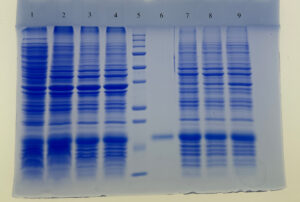
Description:
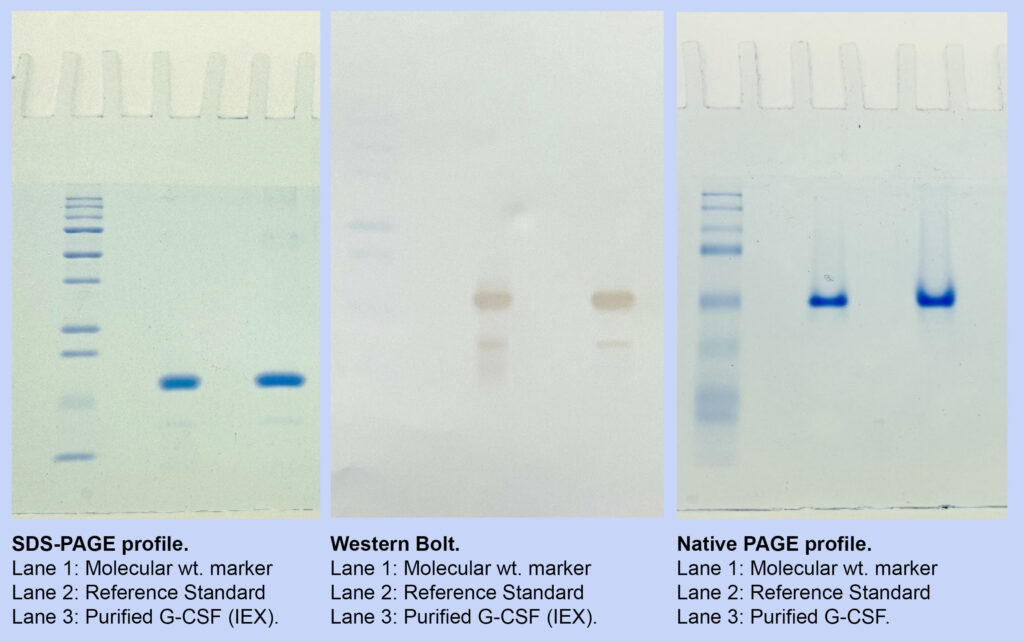
SDS-PAGE profile of expressed protein during biomass and IB processing steps. Molecular weight of the expressed GCSF is aligned to the reference standard used.
Lane 1 : Cell Lysate
Lane 2 – Lane 4 : Biomass sample
Lane 5 : Molecular weight marker
Lane 6 : Reference Standard
Lane 7 – Lane 9 : IB samples
Purification
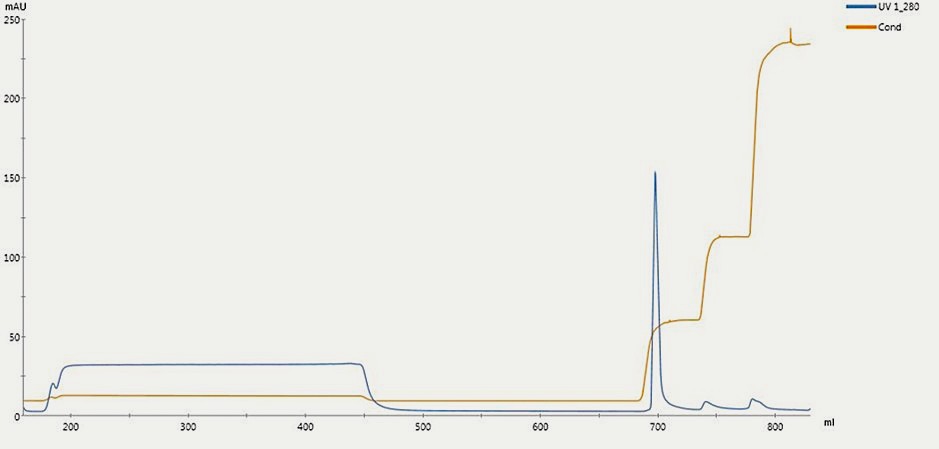
Description:
Chromatogram of G-CSF purification using Ion Exchange (IEX) Chromatography shows a sharp peak indicating purified G-CSF recovery at specific conductivity.
Analytical Test
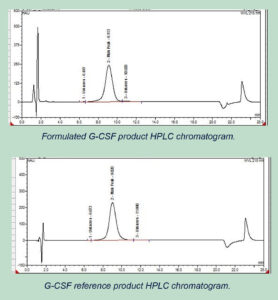
Description:
The HPLC runs for formulated G-CSF product and reference G-CSF sample shows similar Retention Time (Rt) values for the main peak, indicating the identity of the product.
State of the art Biopharmaceutical Manufacturing Unit






